Mapping Key Ethical Issues Surrounding Electroceutical Treatments for Depression
 |
Eleni Varelas, 2nd year |
 |
Marissa Cortright, 3rd year |
Abstract
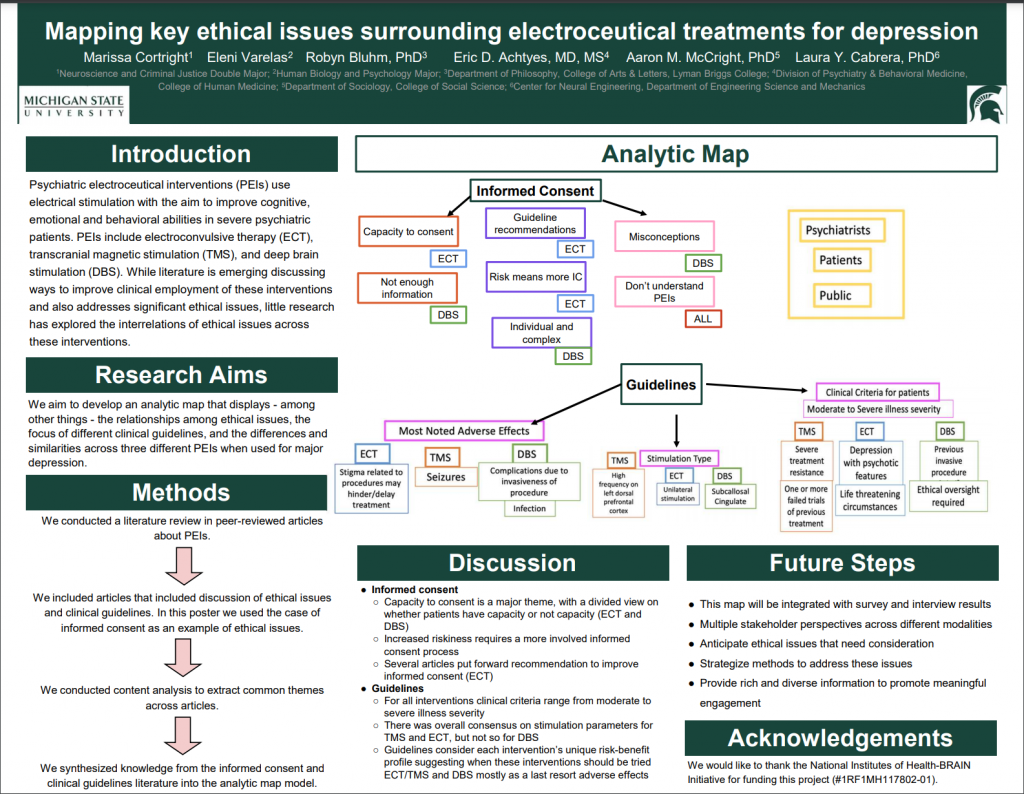
Failure of first-line treatments for some patients with depression has mobilized scientific communities to look toward electroceuticals – interventions which employ electric and magnetic stimulation therapeutically. A growing body of literature investigates how to improve clinical protocols for electroceuticals like electroconvulsive therapy (ECT), transcranial magnetic stimulation (TMS), and deep brain stimulation (DBS) in depression.
While this literature recognizes that electroceuticals raise significant ethical questions, little research has focused on the interrelations of ethical issues across these interventions. We are developing an analytic map to identify shared concerns, interrelations, and differences across interventions. Here we present examples of the analytic map we are developing based on our literature review. To illustrate the benefits of using this approach we present two key areas – informed consent and clinical guidelines.
For example, patient capacity to consent is an overarching argument throughout the electroceutical literature, while patient selection criteria is a topic heavily discussed in clinical guidelines. This map will form part of a larger project that includes the creation of analytic maps from interview results and national survey results with three stakeholder groups. Using this information, we will create a final map that integrates different sources of information, and that accounts for multiple stakeholder’s perspectives and views on different modalities. This map approach can help anticipate ethical issues that need consideration, strategize methods to address these issues, and provide rich and diverse information in a way that promotes meaningful engagement.
Click poster to view full screen.